Abstract
Background: BCL-2 is an anti-apoptotic protein associated with chemotherapy resistance and survival of acute myeloid leukemia (AML) cells following cell stress or DNA damage. Venetoclax (Ven) is a BH3-mimetic that competitively binds to BCL-2, allowing BH3 proteins to bind to pro-apoptotic proteins (BAX, BAK) and induce cell death. The combination of Ven with Azacitidine (Aza), is now standard of care therapy for older patients with AML. Poly (ADP-ribose) polymerase (PARP) inhibitors prevent the repair of single stranded DNA breaks by blocking the nicotinamide adenine dinucleotide (NAD) catalytic domain of the PARP protein and preventing dissociation of PARP from the DNA (termed PARP trapping). The PARP inhibitor, talazoparib (Tala) displays 10,000-fold increased PARP trapping as compared to other clinical PARP inhibitor agents in tumor models. We hypothesized that Tala could enhance the anti-leukemic activity of Ven on AML cells specifically by increasing DNA damage.
Methods: Human AML (Molm13) cells were continuously exposed to increasing doses of Ven (1nM - 10mM), Tala (1nM - 10mM), and Aza (0.1-5mM) alone and in combination. Synergy reports were generated using CompuSyn software. Cell viability was measured using a WST colorimetric assay. Apoptosis (annexin /PI), DNA damage (pH2AX), and cell cycle arrest (propidium iodide) were quantified by flow cytometric analysis. DNA damage was also evaluated using the Fast-Halo assay. In vivo efficacy was assessed in NSG mice systematically engrafted with stably luciferase transfected Molm13 BLIV cells via tail vein injection. Cohorts of mice (5-10) were treated with vehicle, Ven (100 mg/kg given 5 days/week for 3 weeks), Tala (0.33 mg/kg for 5 days/week over 3 weeks), Ven + Tala (same doses), and Ven+ Aza (1.25 or 2.5 mg/kg daily for 7 days). Treatment effects on leukemia burden, toxicity, and overall survival were determined using weekly whole animal bioluminescent imaging, total animal weights, and time to morbidity, respectively.
Results: Human AML cells treated with Ven displayed dose and time-dependent induction of DNA damage, apoptosis, and cell death. Similarly, treatment with single agent Tala resulted in dose-dependent anti-proliferative effects and PARP trapping. Combination in vitro therapy with Ven (0.1 - 1 μM) + Tala (10- 100 nM) or Ven (same doses) + Aza (1 μM) resulted in significant and synergistic anti-leukemic effects improving upon monotherapy with Ven, Tala, or Aza alone. However, in contrast to Ven+Aza, increased levels of DNA damage (as determined by phosphorylated H2AX and Fast Halo assays) were detected as well as induction of apoptosis, cell cycle arrest, decreased viability, and increased cell death vs vehicle and single agent therapy.
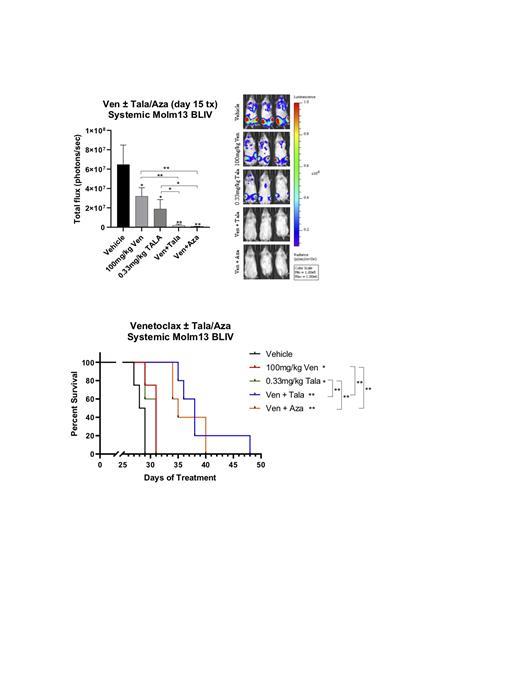
We then evaluated the effects of in vivo treatment with vehicle, Ven, Tala, Ven + Tala, and Ven +Aza in NSG mice systemically engrafted with human luciferase-tagged AML cells (Molm13 BLIV). Single agent Ven or Tala therapy decreased systemic AML burden but did not prolong overall survival over vehicle treated mice. Combination Ven + Tala significantly prolonged overall survival of leukemia xenografted mice over vehicle, Ven, or Tala monotherapy. Of note, Ven + Tala treatment appeared to result in similar disease burden reduction and prolonged overall survival as Ven + Aza treated animals (Figure 1). Preliminary experiments of iv vivo triple therapy with Ven + Aza + Tala in AML xenografted mice resulted in further reduction in AML disease burden but was associated with marked weight loss leading to early morbidity and shortened overall survival (data not shown). Additional doses and schedules of this triplet approach are under investigation.
Conclusions: Our results demonstrate that addition of the potent PARP inhibitor, talazoparib, to the BCL-2 inhibitor, venetoclax, enhances DNA damage and results in potent in vitro and in vivo activity in preclinical human AML models. Further evaluation of this combination in primary AML samples with varying genetic backgrounds as well as in confirmatory primary xenograft models is underway. These data strongly support future clinical investigation of PARP inhibitors as a novel class of agents with the potential to significantly enhance the efficacy of novel targeted therapies for acute leukemia.
Wang: BMS/Celgene: Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Honoraria, Other: Advisory Board, Speakers Bureau; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Consultancy, Honoraria, Other: Advisory Board; Mana Therapeutics: Consultancy, Honoraria; DAVA Oncology: Consultancy, Speakers Bureau; Takeda: Consultancy, Honoraria, Other: Advisory board; Stemline Therapeutics: Consultancy, Honoraria, Other: Advisory board, Speakers Bureau; Jazz Pharmaceuticals: Consultancy, Honoraria, Other: Advisory Board; Kite Pharmaceuticals: Consultancy, Honoraria, Other: Advisory Board; Kura Oncology: Consultancy, Honoraria, Other: Advisory board, steering committee, Speakers Bureau; Novartis: Consultancy, Honoraria, Other: Advisory Board; Rafael Pharmaceuticals: Other: Data safety monitoring committee; Gilead: Consultancy, Honoraria, Other: Advisory board; Daiichi Sankyo: Consultancy, Honoraria, Other: Advisory board; PTC Therapeutics: Consultancy, Honoraria, Other: Advisory board; Genentech: Consultancy; MacroGenics: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal